Bacteriophages: a promising approach to fighting antibiotic-resistant bacteria
By Laura H. Kahn | October 9, 2018
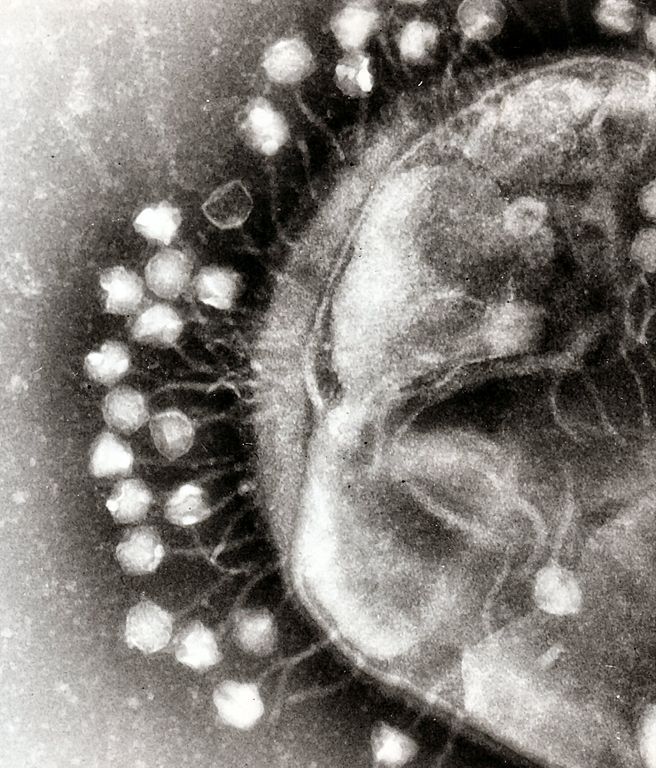 A transmission electron micrograph of multiple bacteriophages attached to a bacterial cell wall, shown at a magnification of approximately 200,000. Photo credit: Graham Beards
A transmission electron micrograph of multiple bacteriophages attached to a bacterial cell wall, shown at a magnification of approximately 200,000. Photo credit: Graham Beards
In 2015, a 69-year-old psychology professor from San Diego, Tom Patterson, became sick with severe abdominal pain, fever, and vomiting while vacationing in Egypt. His condition worsened, and he was medevacked to Frankfurt, Germany, where doctors found a collection of fluid around his pancreas, called a pseudocyst. The fluid was drained and tests showed it to be infected with a resistant strain of Acinetobacter baumanii, a deadly type of bacteria particularly problematic in the Middle East. He went into septic shock and fell into a coma. The bacteria was resistant to all antibiotics.
Patterson’s wife, Steffanie Strathdee, a professor of global health at the University of California, San Diego medical school, searched for alternatives to antibiotics and found bacteriophages. Phages are the most prevalent bioforms (i.e. biologically active nanoparticles) on the planet and are the natural foes of bacteria. Bacterial immune systems evolved to defend against phages. But phages developed countermeasures, meaning that phage evolution can overcome bacterial resistance.
Together with the chief of infectious diseases at UCSD, Strathdee reached out to phage researchers at a variety of institutions in academia, government, and industry to find a phage treatment for his infection. The US Food and Drug Administration (FDA) provided emergency approval for experimental intravenous and intra-abdominal-cavity treatments. The phage cocktail crafted to attack the professor’s infection needed several adjustments, but after three days of treatment in the intensive care unit at Thornton Hospital, part of UC San Diego Health, he woke from his coma. Patterson was eventually cured, returning to good health. Descriptions of the successful phage therapy case are available in the TED talk “How Sewage Saved My Husband’s Life” and in a UCSD news release. The case was also published in an academic journal.
The Patterson case was so revolutionary that UC San Diego established North America’s first bacteriophage therapy center. In addition, the FDA held a two-day workshop discussing the scientific and regulatory issues surrounding phage therapy.
In 2012, I wrote about bacteriophage therapy as one possible solution to worsening antimicrobial resistance, a major threat to human and animal health. Much has happened since then to highlight the antibacterial potential of phages, but unfortunately, minimal federal support and lack of pharmaceutical industry interest have hindered the research and development needed to make widespread bacteriophage therapy (aka “phage therapy”) a reality.
We should take advantage of phages’ evolutionary antibacterial capabilities and make them work for us.
Needed: research funding for phage therapy. In the early 20th century, there was interest in phages in the United States and Western Europe, but that interest vanished when antibiotics—highly effective and easier to use than phages—became available in clinical settings. Phages are highly specific and must be precisely matched to the bacteria they’re meant to destroy; this means the offending bacteria must be identified before doctors implement phage therapy. This feature has its plusses and minuses. While it makes phage therapy harder to use, the need to identify the target bacterium also mandates a level of precision that doesn’t exist with antibiotics. We might consider antibiotics a form of “sloppy” medicine, because they kill bacteria indiscriminately.
As researchers learn more about the importance of the human microbiome in health and disease, they are learning that the microbes that live in and on humans are as important to health and well-being as any organ. Alterations in the microbiome—such as what happens with antibiotic therapy—have been linked with a number of ailments, including autoimmune diseases, obesity, allergies, asthma, and some cancers.
But the level of diagnostic precision needed for successful phage therapy has presented a stumbling block; with the exception of the example of strep throat, rapid diagnostic capabilities are currently insufficient to guide phage therapy, especially in outpatient settings where most antibiotics are prescribed. In seriously ill, hospitalized patients, however, phages could be more readily implemented as important alternatives when antibiotics fail, particularly in treating infections involving the gastrointestinal tract, skin and soft tissues, and the bloodstream.
Unfortunately, the current level of National Institutes of Health-supported research funding on phage therapy is unlikely to advance the science and technology needed to make it a nationwide reality. I conducted an informal search of the NIH’s Research Portfolio Online Reporting Tools (RePORT) of the main category of NIH-funded research projects, designated “R01,” during the agency’s 2016 fiscal year, using the term “bacteriophage.” The search yielded 49 out of a total of 23,664 projects. Of these 49 projects, 21 involved investigating phages’ structures and functions as they relate to bacterial or viral diseases. Of these 21 projects, only two specifically studied phages’ potential as antibiotic substitutes; the sum total of their awards: $968,200. The total of awards for the 21 projects funded in fiscal 2016 was approximately $8.1 million. In that fiscal year, the NIH awarded a total $10.66 billion to 23,664 research grants. Therefore, the 21 bacteriophage projects constituted approximately .076 percent—less than 1 percent—of the total R01 grant awards. The two bacteriophage antibiotic substitute studies constituted .009 percent—less than one-tenth of 1 percent—of total R01 funding in 2016.
Other ways to encourage developments in phage therapy. In addition to minimal governmental research funding, the pharmaceutical industry hasn’t been interested in developing phage therapies because of regulatory and patentability concerns. But these concerns can be readily addressed. Phages adapted to newly evolved, resistant bacteria could be analogous to updated influenza vaccines that get approved each year without undergoing time-consuming Investigational New Drug (IND) approval processes. Novel technologies to isolate, bioengineer, and produce phages at scale could be patented. For example, phages have been engineered to reduce bacterial biofilms.
Phages are generally non-toxic, but since they are derived from environmental sources that humans should not be exposed to—including bacterial debris and toxins—phage preparations must be purified before therapeutic use. Studies are needed to delineate their safety in human and animal use. Also, while some phages kill bacteria—these are called “lytic” phages—others, called “lysogenic” phages, insert their genetic material into the cells and lie in wait, dormant. Lysogenic phages are not useful in responding to resistant bacteria and need to be isolated from phage supplies used in antibacterial treatment. Potential immune responses against phages, especially after repeated intravenous exposures, need to be evaluated, as well.
Safe, effective treatments against deadly, antibiotic-resistant bacteria are essential for the practice of modern medicine. Beyond worsening the existing antimicrobial resistance problem, overuse of antibiotics appears harmful to human microbiomes. Health care providers must minimize their use as much as possible.
Phages have the potential to provide sustainable antibacterial therapies in medicine—but their current technology is about a century old. As an analogy, technologies for renewable energy took decades to research and develop, but those efforts are now coming to fruition with more cost-effective solar and wind power. Phage therapy has the potential to address the problem of worsening antimicrobial resistance. Supporting more projects that advance the science and technology of phage therapies should be a global priority.
Together, we make the world safer.
The Bulletin elevates expert voices above the noise. But as an independent nonprofit organization, our operations depend on the support of readers like you. Help us continue to deliver quality journalism that holds leaders accountable. Your support of our work at any level is important. In return, we promise our coverage will be understandable, influential, vigilant, solution-oriented, and fair-minded. Together we can make a difference.
Topics: Biosecurity, Columnists