A more common enemy
Colorized image of an Aedes mosquito, a transmitter of multiple diseases. (NIAID)
October 28, 2024
Spanish scientists, trying to understand the effects of climate change on human health, recently measured excess deaths in Europe during the summer of 2023, the hottest on record before summer of 2024 exceeded it. They found that more than 47,000 people who wouldn’t have been expected to die did, as relentless heatwaves pushed temperatures in France, Germany, and other countries above 100 degrees.
Heat can kill and sicken directly. It preys on the vulnerable, the elderly, the very young, and those who work outside, among others, causing heat stroke, respiratory issues, cardiac arrest, and other health problems. Beyond these impacts, there is growing evidence of climate change’s influence on yet another factor affecting human health:
Rising temperatures may be leading to major, long-term changes in the epidemiology of infectious diseases.
There is a typical pattern in how global warming affects pathogen epidemiology: Microorganisms move to become endemic (normally present) in areas of more temperate climate and higher altitude. This trend can be seen with many diseases that once were absent or rare in temperate Europe but may be becoming more common.
As they follow their ideal temperatures northward and into higher altitudes, mosquitos and other animals that harbor pathogens may bring West Nile virus, dengue fever, Crimean Congo hemorrhagic fever, and Lyme disease to areas in Europe and elsewhere where those diseases weren’t present before or where they posed less of a threat in the past.
At the same time, pathogens themselves—including vibrio bacteria and the fungi Candida auris— may be evolving, adapting, or changing in response to higher temperatures. They’re becoming more dangerous to people.
The vectors. Changes in land and ocean temperatures force the reconstruction of ecological communities—that is, the arrangement of species that live together and interact with one another. These reconstructions affect the microbiomes of ecosystems, too. Pathogens and organisms, known as vectors, that transmit pathogens onward thrive in specific, unique, temperature ranges.
Mosquitos, which transmit a host of diseases, are among the most troubling of vectors. Mosquito species have temperature ranges in which they thrive; climate change is altering or expanding these ranges.
As temperatures rise, Culex mosquitoes, vectors for West Nile virus, have been spreading. In warming areas, they will be able to replicate more and survive for longer periods of time, leading to an expansion of West Nile virus vulnerability, both in time and space. Outbreaks will start earlier during the year (spring temperatures are considered an accurate predictor of the summer West Nile virus burden), last more months, and affect more geographical regions than before.
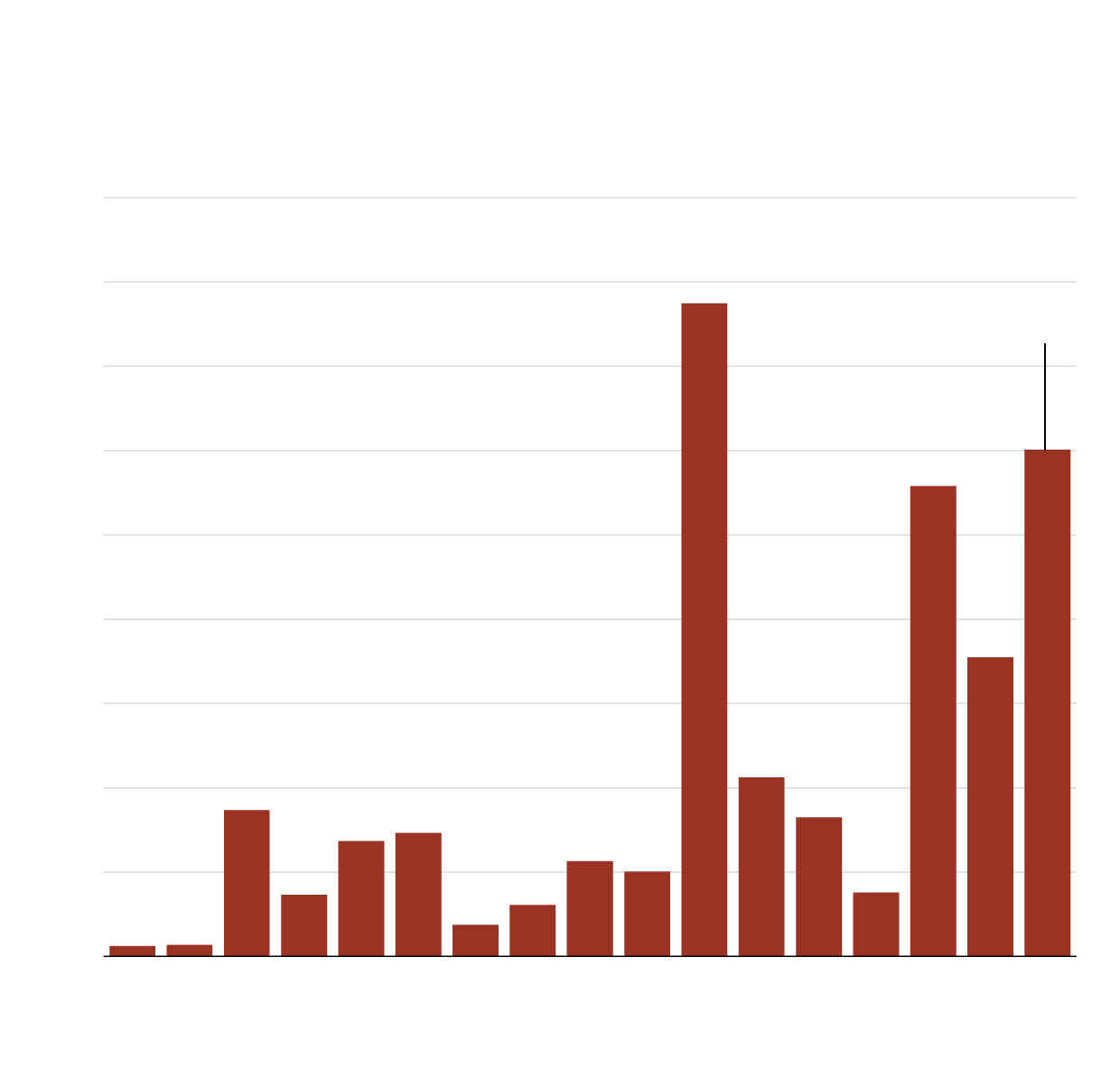
Locally acquired cases of West Nile Virus in Europe
European Centre for Disease Prevention and Control
1,800
1,600
As of Sep. 26
1,400
1,200
1,000
800
600
400
200
0
2008
2009
2010
2011
2012
2013
2014
2015
2016
2017
2018
2019
2020
2021
2022
2023
2024
Locally acquired cases of West Nile
Virus in Europe
ECDC
1,800
1,600
To Sep. 26
1,400
1,200
1,000
800
600
400
200
0
2018
2020
2022
2008
2010
2012
2014
2016
2024
The ECDC includes EU Member States, the European Economic Area, and EU-neighboring states. The UK is included in this data until 2019. Between March 1 and September 26, 2024, 18 European countries had reported 1202 locally acquired human cases of West Nile Virus.
While West Nile virus infections are usually mild or asymptomatic, in some cases, however, they can progress to neurological symptoms and even death. According to 2024 data from the European Centre for Disease Prevention and Control (ECDC), through Oct. 2 there were 88 deaths and 1,202 locally acquired West Nile virus cases (cases are a vast under-estimate since most mild cases are not diagnosed), the highest number for the same period in the past decade, with the exception of the particularly bad 2018 season. More than 30 European regions reported locally acquired infections for the first time ever.
A predominantly climate-induced expansion will make up to an additional 244 million European citizens vulnerable to West Nile virus.
Similarly, increased temperatures will allow the geographical expansion of Aedes albopictus mosquitoes, ecologically flexible and efficient vectors of both dengue and chikungunya viruses. Dengue usually causes a mild infection that may be accompanied by rash, but in certain cases can evolve into a severe hemorrhagic syndrome. Chikungunya typically presents with fever and rash and joint pain, the latter of which may become chronic and debilitating.
Italy has seen two large outbreaks involving hundreds of locally acquired cases of chikungunya virus in 2007 and 2017. Such episodes could become more frequent in the near future.
In June, the European Centre for Disease Prevention and Control reported there had been a significant rise in locally acquired cases of dengue fever in 2022 and 2023, a period in which hundreds of infections were logged. The previous major European outbreak was in Greece in 1928, with more than 1,000 casualties. A pathogen that was absent in many European countries for almost a century appears poised for re-emergence.
A predominantly climate-induced expansion will make up to an additional 244 million European citizens vulnerable to West Nile virus.
The A. albopictus mosquito may be spreading the dengue and chikungunya viruses, but Aedes aegypti is an even more competent vector. While A. aegypti is absent from Europe, with the exception of Cyprus and some Black Sea coastal regions, increased temperatures may eventually change this pattern. If that happens, one can reasonably anticipate a more worrisome scenario for mosquito-borne illnesses in Europe. A. aegypti can also transmit the zika and yellow fever viruses.
Prognostic models have demonstrated that by 2050, 100 to 200 million more people worldwide (compared to today) will reside in areas where A. aegypti is capable of year-round survival, unavoidably rendering these individuals susceptible to the pathogens the mosquito can harbor. Europe will be one of the regions predominantly affected, along with East Asia and North America.
The two mosquito species have different preferred temperatures, with A. albopictus having an optimal temperature curve that peaks lower than that of A. aegypti, 26 degrees Celsius versus 29 degrees Celsius. While a warming world may become less optimal for A. albopictus, that will not counteract the overall effect of climate change on the epidemiology of the diseases these mosquitos carry, as A. aegypti is the better vector anyway. Also this temperature effect on A. albopictus does not involve Europe, where a further increase in endemicity is expected.
SIDEBAR
As climate change leaves fewer animals to infect, will disease-carrying insects target humans?
Since 1900, more than 460 vertebrate species appear to have gone extinct, at least in the wild. That’s a big number. Scientists would have expected only nine such losses. The extinction rate is, moreover, accelerating. Extinction has taken species ranging from passenger pigeons, which may have once composed 40 percent of US birds and darkened skies as they flew in mile-wide flocks, to the thylacine, or Tasmanian tiger, a species that, though extinct since the 1930s, remains so beloved a fan base continues to search the wild for survivors and a biotech company is working to...
Malaria has long been a prolific killer, causing hundreds of thousands of deaths each year. Social factors and well-established preventive interventions like mosquito control and the use of bed nets largely influence malaria epidemiology, and the effect of climate change on the incidence of malaria and its geographical distribution may be less significant than is the case with other mosquito-borne diseases.
Although malaria is associated with warm tropical or subtropical areas, some of these regions may grow too warm for the A.s gambiae mosquito—a primary vector for malaria transmission—to thrive. Areas of high malaria transmission such as sub-Saharan Africa or Southeast Asia may become less suitable for these mosquitos in the future, leading to a decreased burden of disease in the world overall, despite increasing endemicity in sparsely populated rural areas of higher altitude that, by warming up, will become more suitable.
Of course, as the recent 2024 Europe portion of the Lancet Countdown on health and climate change demonstrated, most European countries exhibited an increase in the annual months suitable for Plasmodium falciparum and Plasmodium vivax—two major malaria-causative parasites—transmission in recent decades.
It should be noted that projections of where mosquitos will live and thrive as the climate changes do not take into account the potential for species to adapt. For example, A.s gambiae larvae may exhibit greater tolerance for higher temperatures through a genetic mutation called 2La inversion polymorphism.
Ticks are another prominent vector for diseases, and tick-borne disease is on the rise in some places.
Recent reports from Spain of locally acquired cases have increased concerns that parts of Europe that have so far not experienced a problem with Crimean Congo hemorrhagic fever—a serious tick-borne disease that kills about 50 percent of hospitalized patients—could become more hospitable to the disease.
In the same vein, the endemicity niche of Ixodes ricinus ticks, implicated in human Lyme disease and tick-borne encephalitis, significantly expanded between 2013 to 2022 in comparison to past decades. It now lives in regions of Central and Northern Europe, similar to the northbound trend of tick movement observable in the United States. Many European regions now show an increase in the mean number of months that are optimal for I. ricinus nymphs’ feeding activities.
Changes in pathogens. Higher average temperatures may also directly influence characteristics related to a pathogen’s transmissibility and infectiousness.
Increased oceanic and sea surface temperatures (European seas are warming at four to seven times the global rate) will favor Vibrio bacteria species, pathogens that may induce major morbidity (and mortality) in susceptible populations, particularly people with pre-existing liver disease. Northern European seafronts have implemented surveillance systems to assess the conditions for Vibrio in order to close beaches, with more coastline now suitable for Vibrio infections in countries such as Sweden, Finland, Belgium, Russia, and the Netherlands.
Heat may affect gene expression (the process by which the genetic code is read in order to produce functional parts of a cell) in Vibrio species in ways that allow for efficient infection of humans. (Increased water temperatures may also favor the presence in fresh water of Naegleria fowleri, a dreaded pathogen causing a fatal form of encephalitis. Isolated cases are still recorded in the United States; in Europe, there have been no cases apart from a fatal 2004 case in a boy in Italy.)
In addition to viruses, bacteria, and parasites, climate change may increase the risks posed by fungi. Healthy humans are typically protected from systemic fungal infections due to the pathogens’ inability to survive in human temperatures; infections are usually limited to external parts of the body. But the gradual increase in ambient and oceanic temperatures has allowed for selection of more thermoresistant fungal species, able to also tolerate internal human temperatures and induce systemic disease.
The most valid explanation for the emergence of C. auris—a dreadful and persistent fungal pathogen that simultaneously emerged in the early 2010s in various parts of the world—suggests that it evolved as a human pathogen through just this sort of thermal adaptation, from predecessors located possibly in marine environments.
What should be done? Health literacy about tropical and neglected diseases remains limited, not only for the public but also for legislators and health professionals. This can lead to inadequate containment of a spreading pathogen, delayed laboratory diagnoses, and also inadequate implementation of personal protective measures.
Addressing the risks of climate change on infectious diseases will extend beyond the concept of “one health,” the idea that human, animal, and environmental health are inextricable. Although promisingly broad as a philosophy, in practice, it remains anthropocentric: One health’s aims and proposed actions revolve around the estimated effect that a certain pathogen will have on humans, either human health or the economy if, say, it affects the agriculture and livestock breeding sectors. The concept barely takes into account the overall equilibrium of ecosystems, in which humans are a part and not the epicenter. As some have pointed out, the environment is “the neglected component” of one health.
An optimal response to the climate-related spread of disease demands the coordination of numerous regulatory bodies and ministries. But governmental responses to the growing threat that infectious diseases may pose as the climate changes will likely not follow that optimal pattern. Climate change issues might be handled by an environmental agency, while an agency for agriculture and veterinary issues might handle overlapping issues regarding plants and animals. A ministry of health might deal with the human-related issues of these infections and outbreaks. A lack of coordination is already evident in the case of the US response to avian influenza; states have responded vastly differently to the possibility of H5N1 influenza spread in mammals and humans. For example, there were delays in testing suspected cases of H5N1 in Missouri—a state with no known H5N1 case in animals—when health care practitioners developed respiratory infection symptoms after contact with a confirmed human H5N1 case, a situation that might indicate person-to-person transmission. On the other hand, proactive testing in California has resulted in demonstrating tens of infected cattle herds as well as people infected through contact with them.
The discrepancy in response is indicative of a lack of an effectively implemented nationwide plan as to whom to test and when; which herds to test and when; and whether and how to apply milk surveillance widely. Scientists have also expressed concern on the lack of data available from US Department of Agriculture (USDA).
Region by region, climate change may have opposite effects on infectious disease epidemiology, benefitting, for instance, hantavirus carrying voles in Central Europe, while harming voles in colder Scandinavia. It some cases it may affect pathogens directly through adaptation or changes in gene expression. Or it can, more broadly, affect the structure of an ecosystem, making it easier or harder for vectors or pathogens. Such changes can add synergistically to other climate-based forces, such as in the “mast year” that brings plentiful nuts and therefore rodents to an area, potentially exacerbating a pre-existing northward movement of disease-carrying ticks.
The ways in which climate change will affect pathogens, vectors, or ecosystems will be specific. But in an epoch that some observers have dubbed the 6th Extinction—when the overall impact of anthropogenic changes to the environment are creating myriad habitat disruptions and alterations in the flora and fauna of ecosystems—another more general thought arises: For some pathogens, it may be humans that emerge as the best remaining host.
Together, we make the world safer.
The Bulletin elevates expert voices above the noise. But as an independent nonprofit organization, our operations depend on the support of readers like you. Help us continue to deliver quality journalism that holds leaders accountable. Your support of our work at any level is important. In return, we promise our coverage will be understandable, influential, vigilant, solution-oriented, and fair-minded. Together we can make a difference.
Keywords: Infectious disease, bacteria, fungi, mosquitos, parasites, ticks, viruses
Topics: Biosecurity, Climate Change