Antimicrobial resistance: an underrated biological threat
By Saskia Popescu | November 21, 2017

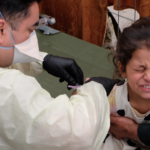
Alexander Fleming, who discovered penicillin, predicted an age when the efficacy of antibiotics would diminish and wither away. Sadly, it seems as if we’re nearing that point. Highly virulent and deadly outbreaks of multi-drug-resistant organisms are becoming increasingly common. Even prestigious hospitals, like the US National Institutes of Health Clinical Center, are not immune to the spread of resistant organisms. Within the United States alone, at least 2 million people become infected with drug-resistant bacteria and at least 23,000 die every year as a result of such infections. Globally, an estimated 700,000 people die from antimicrobial resistance (AMR) annually. Despite efforts by the World Health Organization’s Global Antimicrobial Resistance Surveillance System and other groups, it has been hard to paint an accurate picture of the problem.
Predictions for the coming decades are even more concerning. In 2014, the United Kingdom’s Prime Minister commissioned a report by economist Jim O’Neill, who chairs the Review on Antimicrobial Resistance, to assess the global impact of antimicrobial resistance and recommend concrete actions. Released in 2016, the report estimates that, without policies to stop the spread of AMR, there will be 10 million deaths annually due to drug-resistant infections by 2050, and a cumulative $100 trillion economic impact.
Potential new treatments face significant economic obstacles, but perhaps an even bigger challenge is the normalization of AMR. Although drug resistance is already wreaking havoc, it receives less attention and funding than threats such as emerging natural diseases, biological weapons, and synthetic biology. By any definition, antimicrobial resistance should qualify as a global catastrophic biological risk that rivals these other threats.
Roadblocks and roadmaps. Despite the existing burden of AMR, and the alarming estimates about its future, there are significant hurdles and roadblocks to antimicrobial development and stewardship. The process of discovering, developing, and bringing to market a new drug is astoundingly expensive and challenging. In an era when antimicrobials are no longer big money-makers, it’s not surprising that there hasn’t been a new class of antibiotics discovered since 1984. Antibiotics developed in recent years have been variations of existing drugs. This lack of antibiotic innovation means that the strategies drug developers are using to fight bacteria are only slightly different than historical ones, and bacteria that are already resistant to one antibiotic typically become resistant to a whole class of antibiotics.
In response to this challenging market and the increasingly desperate need for new medical countermeasures and strategies, public and private forces have been struggling to find incentives to stimulate drug discovery. Private efforts include The Pew Charitable Trusts’ campaign to combat AMR by investigating barriers to drug discovery, and providing approaches to overcome both scientific and organizational obstacles to the successful development of new antibiotics. A May 2016 Pew report identified several needs: a framework to share information and expertise across the research community, a targeted approach to tackling basic scientific barriers (for example, a lack of training), and tools that can evaluate alternatives to traditional antibiotic use.
A program called CARB-X (short for Combating Antibiotic Resistant Bacteria Biopharmaceutical Accelerator), created as a public-private marriage between the US Biomedical Advanced Research and Development Authority and the UK charity Wellcome Trust, is a new strategy to accelerate antimicrobial development during the next 25 years, and to reduce the barriers to market entry. What makes CARB-X so exciting is that it supports start-ups and small companies that have discovered potential new antimicrobial products but can’t afford the human testing required to bring them to market. CARB-X steps in, not only with funding but also with professional guidance.
One significant roadblock to combatting AMR is the misuse and overuse of antimicrobials, which include antibiotics. The Centers for Disease Control and Prevention estimates that 30 to 50 percent of the antibiotics prescribed in US hospitals are unnecessary or inappropriate. With surveillance efforts lagging, reports like those from Pew help shed light on the prescribing practices that fuel AMR, and suggest antimicrobial stewardship strategies to reduce the misuse of antimicrobials.
The Pew report evaluated antibiotic prescribing practices in outpatient settings such as urgent-care and dental clinics. The researchers found that in 2014, outpatient health care providers within the United States wrote 266 million antibiotic prescriptions, which amounted to roughly 835 antibiotic prescriptions per 1,000 people. Of these outpatient antibiotic prescriptions, 44 percent were for acute respiratory conditions, and half of these prescriptions were unnecessary—because many respiratory conditions are caused by viral infections that are not susceptible to antibiotics. Establishing antimicrobial stewardship strategies requires understanding why and where medical providers are prescribing antibiotics.
International partnerships are also taking action on antimicrobial resistance. It is number one on the action list of the Global Health Security Agenda, for example, which has more than 50 members ranging from states to non-governmental organizations. This prioritization highlights not only the importance of the problem, but also the desperate need for coordinated global efforts to combat it. Antimicrobial resistance can emerge from a range of sources: drug use in agriculture, overprescribing and improper use in humans, and even medical tourism. It is logical that the response efforts should be just as diverse.
A normalized threat. One of the biggest impediments to developing effective treatments is the normalization of AMR. Researchers, infection prevention and control practitioners, and medical professionals have been raising the red flag for decades. Drug-resistant infections, such as methicillin-resistant Staphylococcus aureus (MRSA), used to be rare events in health care but are now considered a common occurrence.
There’s an old saying in infectious disease medicine and epidemiology: “When you hear hoof beats, think horses, not zebras.” In other words, the correct diagnosis is more likely to be a common disease than a rare one. But in medicine and public health, biodefense and biosecurity efforts tend to focus on “zebras” such as Ebola, anthrax, and smallpox that can spread quickly, do great harm, and cause panic. The problem is that AMR never really had a zebra phase, and now it is a horse that has long been out of the stable.
AMR poses a national security threat due to its ease of transmission and its potential for a major public health crisis. Unfortunately, the spread of highly resistant diseases has received far less concern and funding than emerging infectious diseases. AMR isn’t as sexy or flashy as a disease such as Ebola, but it is just as sinister and should be considered and addressed as the pandemic that it is.
Normalizing the threat of antimicrobial resistance has allowed it to spiral out of control. Between 2000 and 2010, antibiotic usage grew by 30 percent worldwide. In the United States, four out of five Americans are prescribed antibiotics each year, and one-third of these prescriptions are unnecessary, according to the Centers for Disease Control. AMR adds $20 billion in direct health care costs and an additional $35 billion in lost social productivity.
A global catastrophic biological risk. All of these numbers should put AMR in the category of an ongoing global catastrophic biological event. The cost of the Ebola outbreak in the hardest-hit West African countries is well below the annual burden of AMR in the United States alone. And despite international efforts to contain resistance, there is still no successful global surveillance mechanism for an infectious disease threat that experts have known about for decades.
A Johns Hopkins Center for Health Security report on a working definition for global catastrophic biological risks has pointed to the Black Death and a wide-area anthrax attack on a major city as risks of this caliber. AMR should also be included in this working definition. Not only does it fit the criterion of causing sustained catastrophic damage to national governments and economies, but it is already killing a significant percentage of people.
Global catastrophic biological risks are likely to be sudden developments that are unresponsive to medical countermeasures. AMR was a sudden development decades ago, with the first cases of resistance to the antibiotics tetracycline and penicillin, and it continues to generate new developments—such as the finding of patients in the United States and China who have one of three mcr genes that make bacterial infections resistant to the antibiotic of last resort, colistin.
Biological weapons and outbreaks of natural diseases are undoubtedly threats that society must continue to prepare for, but AMR is already a biological threat – one that is caused by a range of culprits and therefore may be more difficult to overcome. Even global efforts, like the World Health Organization’s recommendations regarding antimicrobial use in agriculture, have failed to address the complexities of microbial resistance. Chavonda Jacobs-Young, Acting Chief Scientist of the US Department of Agriculture, said that the recommendations “erroneously conflate disease prevention with growth promotion in animals” and “are not supported by science,” which points to the gap in international strategies and efforts.
Not only is AMR a global issue, but the contributing factors are diverse and require multiple industries to change common practices, like antibiotic usage in agriculture or limiting antibiotic prescribing in health care. Changing prescribing practices in the United States alone is challenging, but imagine doing so in a country like India, which has virtually unchecked prescribing habits.
Further complicating matters, multiple diseases have become resistant to antimicrobials, and they have different transmission mechanisms. Even more challenging, the spread of AMR is fueled throughout health care, whether it be a long-term care facility or an intensive care unit. Deadly outbreaks within hospitals are obvious indicators that drug-resistant organisms are prolific throughout the health care system. Hospitals, which act as amplifiers by exposing vulnerable patients to these organisms, struggle to contain such events.
While many health security experts look to synthetic biology and emerging infectious diseases as sources for biological threats, AMR is a biological threat that challenges prevention and control efforts across multiple sectors and industries with no clear-cut solution. The battle against resistant bugs requires a novel approach, and that means looking at it as a global catastrophic biological risk capable of triggering a massive pandemic event.
Together, we make the world safer.
The Bulletin elevates expert voices above the noise. But as an independent nonprofit organization, our operations depend on the support of readers like you. Help us continue to deliver quality journalism that holds leaders accountable. Your support of our work at any level is important. In return, we promise our coverage will be understandable, influential, vigilant, solution-oriented, and fair-minded. Together we can make a difference.
Topics: Biosecurity, Voices of Tomorrow