The new science that could help spot the next pandemic before it begins
By Yong-Bee Lim | September 30, 2022
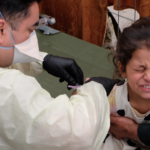
A cough, fever, pain, weakness, and difficulty breathing—those were the 41-year-old’s symptoms when he was admitted to a Wuhan hospital in late December 2019. The previously healthy man had fallen ill six days earlier, according to a paper in the journal Nature, but tests for influenza and other infections were turning up negative. Scientists had a mystery on their hands. Solving it by identifying the cause of the man’s disease would be a first step toward taming the outbreak cropping up in Wuhan that winter. And thanks to cutting edge genome sequencing technology, researchers completed that task with remarkable speed.
A team in Shanghai analyzed the sample from the Wuhan patient in less than two days, using “the latest high-throughput sequencing technology,” to parse out unknown pathogens from a stew of genetic material in a sample. What they found: a genetic sequence highly similar to a bat SARS-related coronavirus, the pathogen that we now know of as the virus that causes COVID-19.
Thanks to evolving technologies like the kind used to identify SARS-CoV-2, scientists have increasing abilities to spot pathogens involved in outbreaks sooner and to track them as they progress. The speed with which researchers pegged the pathogen responsible for COVID-19 stands in stark contrast to that of the first SARS outbreak in 2002, when researchers identified a coronavirus by first isolating it from cell cultures. A CDC timeline of the outbreak says a novel coronavirus wasn’t identified as the suspected culprit behind SARS until late March 2003, four months after cases of unexplained pneumonia emerged in China and nearly a month-and-a-half after Chinese health officials alerted the WHO of the outbreak. For COVID-19, it took a little over a week from when Chinese authorities alerted the WHO for researchers to sequence SARS-CoV-2 and widely publicize their findings.
Advances in bio surveillance and characterization methods and a deeper understanding of how pathogens interact with human hosts mean that governments have more capabilities than ever to detect novel pathogens before they cause pandemics. That’s important because climate change, ecological degradation, and geopolitical tensions mean we’ll likely have to confront more new disease threats as these factors intensify.
Going from the known to the unknown. Currently, the United States and the global community use lists of known pathogens and biologically derived toxins to help prioritize defensive research, counterterrorism, and law enforcement efforts. While this has helped countries and organizations allocate scarce resources towards targeted research on pathogens that are highly lethal, exceptionally infectious, or exhibit other traits that cause immense concern for public health and national security, there is growing worry that a focus only on the known realm of biological threats does not account for biological events that may emerge from unknown organisms that may be circulating in nature, be worked on in labs, or be engineered by state or non-state actor adversaries.
Therefore, researchers and policymakers are trying to find ways to identify and characterize novel pathogens and better understand how disease agents affect biochemical pathways in humans and animals. These technologies could accelerate public health and defense capabilities to detect, identify, and respond to both known and unknown biological threats. Ideally, using them would allow us to detect and respond swiftly enough to mitigate or even prevent a disease from emerging at all.
Detecting a potential pathogen of concern is the first key component of this approach. We can already do this somewhat reliably for known pathogens using detection assays—tests that help identify a known pathogen in a sample. Assays fall under two main categories, which include immunoassays (checking a human sample’s antibody response to specific antigens) and molecular assays (amplifying specifically identified viral genomic material found in a sample, if it exists in the sample, through methods such as PCR tests).
In the case of a novel pathogen, the above options would not necessarily work, because novel pathogens are, by definition, unknown. Fortunately, advances in the life sciences and computational capabilities now allow us to understand and identify the genetic building-blocks of pathogens in new ways. One well-discussed method, known as metagenomic next generation sequencing, can sequence all nucleic acids—DNA and RNA—in a sample and use this information to “simultaneously identify genetic material” across entirely different types of organisms. This approach not only identifies known pathogens, but also potentially novel pathogens by matching them for similarity with databases full of pathogen genomes. Portions of a new coronavirus in a sample might, to some extent, match with catalogued portions of previously identified and sequenced coronaviruses in a database, like how the RNA in the Wuhan patient’s sample was found to largely match a bat SARS-like coronavirus.
After researchers identify a novel pathogen, they need to characterize it. Characterization is the process of understanding how the genetic structure of a pathogen affects its physical characteristics. It allows scientists to identify key attributes, which then offer unique clues or targets to apply towards diagnostics and treatments for the pathogen.
Historically, scientists grew pathogens in different environments in a lab, ranging from cell cultures to animal studies, to discover observable, detectable physical traits, including the presence of toxins or antibiotic-resistance genes. However, these methods can be both time-consuming and expensive. During the COVID-19 pandemic, supply chains for animals became snarled, complicating lab efforts.
Progress in biotechnology and computational biology offers alternatives to more traditional approaches. One such approach relies, again, on applications of sequencing technology—this time to characterize parts or the entirety of a pathogen’s genetic material. This can yield significant insights into how the genetic information of an organism (its genotype) and the organism’s interaction with its environment contribute directly to a pathogen’s observable physical properties (its phenotype) and falls within an area of biological and information studies known as bioinformatics. Combined with ever-more thorough and accurate techniques such as whole-genome sequencing, scientists have tools that can characterize the entire genetic code of an organism in a streamlined process. In this way, they may gain an understanding of what the pathogen is capable of—and what methods might work to thwart it—without extensive laboratory testing.
Whole-genome sequencing has significantly matured thanks to progress in computational power and the rapidly diminishing economic costs necessary to sequence an entire genome. Used historically as a research tool, scientists and researchers have started using whole-genome sequencing for clinical applications in areas such as cancer research, as well as in public applications such as the CDC’s PulseNet network to help “detect and investigate food-borne outbreaks.” However, characterization is still limited to geographic areas that have significant technological capabilities and those countries that can afford whole-genome sequencing costs, which ran close to $1,000 per run in 2016.
While identification and characterization are two necessary capabilities in addressing novel pathogens, scientists have also realized that focusing on pathogens is not the only way to bolster defenses to new diseases. An alternate method is gaining traction: understanding the characteristics of various infections within a host, regardless of whether a pathogen is known— an idea called “threat-agnostic” biodefense. According to researchers at the Pacific Northwest National Laboratory, if health officials using this method encountered a disease threat, instead of referencing a list of harmful pathogens like the US Government’s Select Agent List, and asking “what is it,” they would ask, “is it harmful, and how harmful is it?”
Threat-agnostic biodefense focuses on using cutting-edge techniques to measure, analyze, and identify “common patterns of infection and disease” within animal and human hosts, according to the national lab. If scientists can identify the signals of infection with viruses or bacteria on a cellular level, they could develop countermeasures or diagnostic and surveillance methods for novel pathogens.
The method rests on identifying so-called “bioagent-agnostic signatures”—for example, say, molecules that indicate the presence of bacteria. These signatures would include various data “that describe host characteristics and responses or broadly identify the presence of a class of pathogen,” the national lab researchers wrote in 2021.
While there is much more work to be done to understand the biochemical changes within a host to the degree necessary to make threat-agnostic biodefense a broadly applicable approach, there have already been advances that highlight its potential. In 2021, for example, Israeli researchers won Food and Drug Administration approval for a test to differentiate between bacterial and viral infections—an important distinction for clinicians to make in determining how to treat a disease.
The advantage of threat-agnostic biodefense is that it effectively shrinks the near-infinite landscape of biological threats by narrowing the focus to specific pathogen-host interactions—interactions that allow only a limited number of ways for pathogens to enter a host’s cells, hijack cellular machinery, replicate within cells, and escape from cells to propagate further. However, a significant amount of basic research needs to be done to systematically map, understand, and identify bioagent-agnostic signatures. Even as knowledge on bioagent-agnostic signatures grows through further research, hosts may differ significantly in their response due to different biochemical profiles arising from conditions such as past infection exposures, as well as health conditions that may change an individual’s immunological response. Therefore, a generalizable model may be difficult to achieve in the near-term.
As the world continues to face biological event after biological event, significant action must be taken to curtail the worst potential outcomes. New and evolving technologies leveraged towards pathogen-agnostic approaches to biodefense and public health may offer new ways to detect, characterize, and mitigate the risks associated with the emergence of novel pathogens in a wide variety of settings, from farm fields and cities to overseas military bases and hospitals. Some useful technologies and ideas—like pathogen agnostic biodefense–are still in their infancy, others, like whole-genome sequencing remain costly and out of reach, especially in poorer countries.
Taken together, these new methods and technologies for identifying and characterizing novel threats have a significant way to go before they can begin to fulfill their potential promise, but the US government and others should invest in them. Fighting the next potential pandemic pathogen could depend on doing so.
Together, we make the world safer.
The Bulletin elevates expert voices above the noise. But as an independent nonprofit organization, our operations depend on the support of readers like you. Help us continue to deliver quality journalism that holds leaders accountable. Your support of our work at any level is important. In return, we promise our coverage will be understandable, influential, vigilant, solution-oriented, and fair-minded. Together we can make a difference.
Keywords: COVID-19
Topics: Analysis, Biosecurity