A coronavirus vaccine: China’s got one, Russia does, too. Will Trump rush one out?
By Matt Field | August 27, 2020
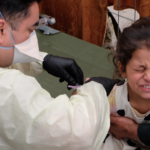
 Scientists say the Trump administration on Sunday authorized a COVID-19 treatment that hasn't been shown to have the benefits the administration is claiming. Experts are growing increasingly concerned the Trump administration is putting its political interest ahead of science as it pushes through COVID-19 medical products. Credit: White House.
Scientists say the Trump administration on Sunday authorized a COVID-19 treatment that hasn't been shown to have the benefits the administration is claiming. Experts are growing increasingly concerned the Trump administration is putting its political interest ahead of science as it pushes through COVID-19 medical products. Credit: White House.
Even as the squiggly red line of coronavirus cases has begun trending down again following a summer of record high case numbers in several US states, the pandemic situation still remains largely grim—schools facing outbreaks, fears of a second wave. There is, however, one bright spot amid the gloom: the prospect that a vaccine gets distributed, in record time. The question experts have begun to ask, though, is, will an inoculation arrive too soon, before it’s been properly tested?
There are reasons to worry the Trump administration may push through a vaccine before it’s undergone complete clinical testing. Rival nations like China and Russia have already announced they’ve got vaccines. The number of COVID-19 deaths in the United States keeps rising. And the presidential election is just two months away. Health experts worry these factors put pressure on the administration to prematurely roll out a vaccine.
Paul Offit, an infectious disease expert and director of the Children’s Hospital of Philadelphia’s Vaccine Education Center, says the upcoming election and the foreign announcements are increasing the heat on the US administration: “I think there’s pressure from countries like China, Russia, even arguably the United Kingdom, where there may be a vaccine that rolls out in those countries before one rolls out here.” A vaccine, he says, should only be released after meeting a high standard of safety and efficacy, the same standard, he says, vaccines have been held to “for the last 70 years.”
Americans disapprove of President Donald Trump’s handling of the coronavirus pandemic by an almost 20-point margin according to poll averages calculated by FiveThirtyEight. Trump has acknowledged a vaccine announcement ahead of the Nov. 3 vote “wouldn’t hurt.” A shot, literally, may represent Trump’s only figurative shot of turning his ratings on COVID-19 around, something health experts are worry about.
“It’s quite apparent that President Trump wants to use treatment or vaccination options as a push for his campaign in November,” says Saskia Popescu, an epidemiologist and professor at George Mason University’s Biodefense Graduate Programs. Political influence on the vaccine development process would be “extremely dangerous,” she says.
Earlier in the pandemic, scientists criticized the administration’s authorization of the anti-malarial drugs hydroxychloroquine and chloroquine, saying there wasn’t evidence that the drugs worked for COVID-19 patients. The Food and Drug Administration (FDA) eventually pulled those authorizations in June, saying the drugs probably aren’t effective and are potentially unsafe. On Sunday, the eve of the Republican National Convention, the FDA authorized another experimental coronavirus treatment, the blood plasma from recovered COVID-19 patients. Critics say the FDA and Trump, who stood alongside FDA Commissioner Stephen Hahn and Health and Human Services Secretary Alex Azar at the announcement, vastly oversold the benefits of the plasma treatment. Hahn ended up acknowledging he hadn’t accurately characterized them.
The FDA’s controversial emergency use authorizations set “a bad precedent because the job of the FDA is to protect the American public, not please the president,” Offit says. The emergency use authorization procedure allows the FDA to authorize unapproved medical countermeasures or unapproved uses of approved drugs and devices during an emergency such as the pandemic in order to diagnose, treat, or prevent diseases and conditions.
Do the administrations recent controversial decisions raise the risk of it authorizing a vaccine before it’s been fully tested in a large Phase 3 clinical trial? “Yes,” Offit says. “There are 1,000 people dying every day in this country. We need a vaccine as quickly as possible, but that’s the one corner you can’t cut; you can’t truncate a Phase 3 trial.” Three of US government-backed vaccine candidates are in Phase 3 trials. Ordinarily, the vaccine development process takes years.
But, Offit says, a vaccine trial could legitimately be cut short by an independent expert group that’s monitoring the study if there is a strong indication the vaccine is effective. “There’s a safety monitoring board for these trials,” Offit says. “If it comes to be that there’s a clear efficacy signal and that there’s no negative safety signal, and you’re only, say, 20,000 people into a 30,000-person trial and the data safety monitoring board says, ‘I think we need to get this vaccine out there now,’ fine. That’s fine.”
An emergency use authorization might be the route the FDA takes to authorize the use of a coronavirus vaccine, Hahn and his colleagues wrote in an editorial earlier this month. The FDA’s Vaccines and Related Biological Products Advisory Committee, which Offit sits on, will need to review the data on a vaccine, they wrote, “to ensure clear public understanding of the evidence supporting vaccine safety and efficacy.”
That promise of transparency, however, hasn’t alleviated the concerns experts have over the FDA’s approval process, and the administration’s authorization for convalescent plasma on Sunday only exacerbated those worries.
“The most recent announcement was the most troublesome, because it seemed like it was being politicized,” says Seema Mohapatra, a law professor and health law expert at Indiana University. “The release was unlike other press releases that the FDA puts out. It looks a lot more like a campaign-type ad.”
At the Sunday press conference about convalescent plasma, Trump announced, “Today I’m pleased to make a truly historic announcement in our battle against the China virus that will save countless lives,” using, as he often does, a racist term for the SARS-CoV-2 virus. While Trump said the plasma is proven to reduce mortality by 35 percent, the Mayo Clinic study that backed the FDA’s emergency use authorization hinted, rather, at a potential benefit to patients who received plasma with a lot of antibodies, relative to those who received plasma with less. The study didn’t compare patients who had received the convalescent plasma to those who hadn’t.
Mohapatra worries that the perceptions of politicization that the FDA’s emergency authorization decisions have had to date could spill over into how people view the authorization of an eventual vaccine. “There’s a concern that when there is a [COVID-19] vaccine, if it is, you know, literally warp speed, kind of rushed to market to coincide with the election, that people are not going to have faith that the vaccine is effective, that people are not going to actually sign up to take the vaccine,” she says.
Trump has done little lately to put critics at ease about whether his administration would inject politics into the vaccine development process. As the president sees it, politics and the machinations of his political enemies are what’s holding back vaccines and treatments for the coronavirus in the first place. In a tweet on Saturday, Trump wrote the FDA or the “deep state” was making it hard for vaccine developers to get the test subjects they need, in order, he argued, to “delay the answer until after November 3rd.” In the Twitter missive, Trump tagged his FDA head, as if to ensure the commissioner got the message.
Trump made a similar point about the FDA slow-walking COVID-19 therapies at the press conference the next day. This time, however, Hahn stood right at his side.
Message received?
Together, we make the world safer.
The Bulletin elevates expert voices above the noise. But as an independent nonprofit organization, our operations depend on the support of readers like you. Help us continue to deliver quality journalism that holds leaders accountable. Your support of our work at any level is important. In return, we promise our coverage will be understandable, influential, vigilant, solution-oriented, and fair-minded. Together we can make a difference.
Keywords: Coronavirus, Donald Trump, FDA, Stephen Hahn, convalescent plasma, emergency use authorization, hydoxychloroquine, vaccines
Topics: Biosecurity