The known knowns, known unknowns, and unknown unknowns of COVID-19
By W. Ian Lipkin | July 21, 2021
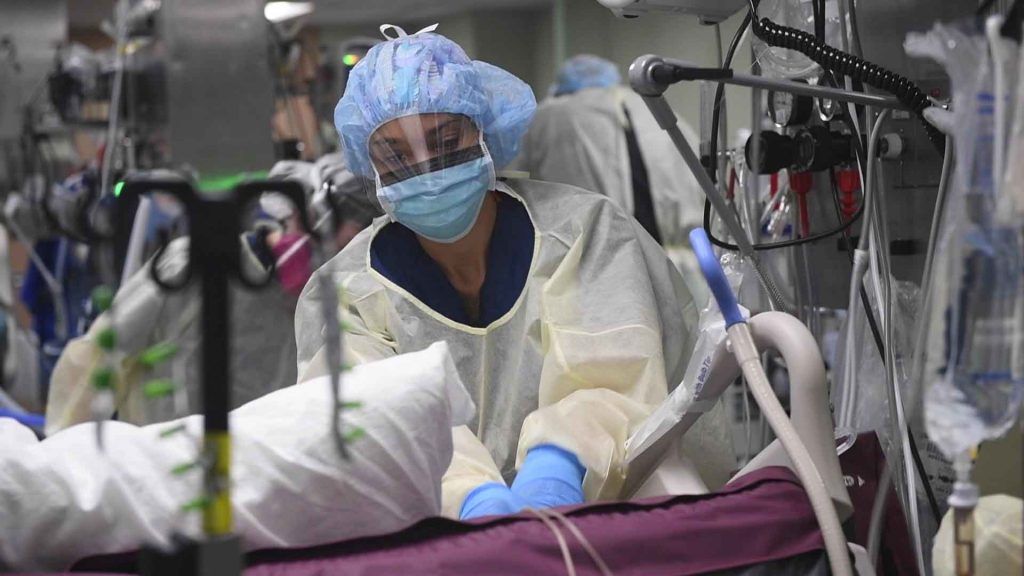 A nurse cares for a patient on a US Navy hospital ship during the COVID-19 pandemic. Credit: US Navy.
A nurse cares for a patient on a US Navy hospital ship during the COVID-19 pandemic. Credit: US Navy.
In 2002, while making the case for the US invasion of Iraq to those who asked for evidence of what we now know to be nonexistent weapons of mass destruction, Donald Rumsfeld referred to known knowns, known unknowns, and unknown unknowns. Nearly two decades later, we are in similar territory in discussing the origin of SARS-CoV-2, the virus that causes COVID-19. Then, as now, we should be wary that incomplete data and strong opinions not determine high-impact decisions.
The known knowns are that SARS-CoV-2 is a new, highly transmissible virus that has the capacity to evolve rapidly. Genetic analyses indicate that closely related viruses existed in wildlife before human disease was detected in the Chinese city of Wuhan in 2019. We also know that despite promises to shut down wild animal markets in the aftermath of the first SARS outbreak in 2003, thousands of animals were sold in such markets in Wuhan. Many of these species are known to be infected and/or infectable with SARS-like coronaviruses. These animals could have been vectors for carrying SARS-CoV-2 to humans and for adaptation to growth in humans.
We also know that the Wuhan Institute of Virology and the Wuhan Center for Disease Control and Prevention collected specimens from wildlife that typically carry coronaviruses and pursued gain-of-function experiments. The level of biocontainment for these experiments may have been inappropriate. Nonetheless, there is no evidence that SARS-CoV-2 was deliberately created. Indeed, the features of SARS-CoV-2 that have resulted in at least four million deaths, lingering disease in millions more, and an estimated $22 trillion loss in output through 2025, could not have been predicted even had someone wanted to engineer a highly transmissible and pathogenic coronavirus and had the tools to do so.
There are many known unknowns. Unlike smallpox, Ebola, or other potentially lethal infectious diseases that announce themselves with a distinctive clinical syndrome, SARS-CoV-2 has the capacity to infect people and cause no or only mild disease. Furthermore, even people who ultimately develop severe disease may be infectious before they have symptoms. This insidious aspect of SARS-CoV-2 biology makes it difficult to track its spread and to determine when, where, and how it first emerged. One clue to the advent of SARS-CoV-2 might have been a spike in the number of cases of respiratory disease or in the use of prescription or over-the-counter medications to treat them. However, there is no evidence of either in Wuhan in the weeks prior to the first documented cases of COVID-19 in November 2019. Wuhan is also a major transportation hub. The first cases may have been imported from other regions in China or throughout the Asian continent where related viruses were reported.
A resource that epidemiologists frequently use for determining the origin and timing of the introduction of a new infectious agent is serially collected samples that can be tested for the presence of viral proteins or genetic material (in this case, RNA), or for antibodies that are produced in response to infection. While investigating the origin of MERS-CoV in 2012, a team of expert researchers of which I was a member found large amounts of viral RNA in young dromedary camels. Additionally, we found high levels of antibodies in blood of more than 75 percent of contemporary adult camels and in blood from camels that had been collected annually and stored for a period of more than 10 years. These findings—plus the presence of viral RNA in camel meat in abattoirs—were helpful in implicating camels as important sources of human infection and in dating the incursion of MERS-CoV into the Arabian Peninsula.
To date, we have not succeeded in similar studies in searching for the origins of the COVID-19 pandemic. The presence of SARS-CoV-2 RNA would be considered unequivocal evidence of the presence of the causative agent. We are rapidly closing in on two years since the beginning of the Wuhan outbreak. The challenge in looking for RNA in archived materials is that it rapidly degrades unless samples are stored at ultralow temperatures. Proteins and antibodies are less labile but are also less specific. Through a mechanism known as cross reactivity, infection with a different, but related coronavirus (like SARS-CoV-1, the causative agent of the 2002-2003 SARS outbreak) can yield results that are mistaken to represent COVID-19. This was not a problem in Saudi Arabia, because no similar MERS-like viruses were circulating in camels or people. However, in the context of the COVID-19 pandemic, we must have assays that distinguish between SARS-CoV-2, SARS-CoV-1, and other coronaviruses.
There is considerable uncertainty about when SARS-CoV-2 first appeared both inside and outside of China. The South China Morning Post has reported the retrospective diagnosis of infection in a 55-year-old man in Hubei province on November 17, 2019. A US Centers for Disease Control and Prevention study of more than 7,000 routine blood donations collected from December 13, 2019 through January 17, 2020 revealed the presence of antibodies to SARS-CoV-2 in at least 84 people in nine US states. Given that an antibody response cannot be detected until two to three weeks after infection, we can only conclude the virus was circulating in the United States for several weeks before the first case of COVID-19 was diagnosed on January 19, 2020. Even more compelling evidence of early infection outside of China was found in a skin biopsy obtained in November 2019 from a woman in Milan, Italy with an inflammatory rash that was found to contain both SARS-CoV-2 viral RNA and proteins. Mathematical models based on studies of viral sequences and evolutionary rates suggest that SARS-CoV-2 emerged in China in early October to mid-November. We do not have the samples needed to search for evidence of infection in humans, wildlife, or domestic animals in China prior to November 2019. We also do not know whether such samples exist or whether the problem is lack of access.
Let us return to what we do know. Three quarters of emerging infectious diseases originate in wildlife. Over the past 40 years, I have personally been involved in addressing several: HIV/AIDS, West Nile encephalitis, SARS, MERS, Lujo, Lassa, Nipah, Dandenong, Ebola, Marburg, dengue, monkeypox, Zika, influenza, and COVID-19. Estimates of numbers of unknown viruses lurking in mammals range from 320,000 to 1,000,000. If even 1 percent of them can infect humans or domestic animals, we may be ignorant of thousands of potential threats to human health and food security. In an increasingly interconnected world, diseases that might once have been contained to a region are now global. Accordingly, the international community can have zero tolerance for wildlife markets and wildlife trafficking for food, medicinal, or pet trade purposes. Our current focus in on China. However, trafficking in wildlife is a global threat and should be banned everywhere. It may have contributed to the emergence of HIV/AIDS and to outbreaks of Ebola and Marburg. France plays a central role in wildlife trafficking, with more than 28 million specimens imported between 2008 and 2017, including 4,000 seizures of illegal transport during that time period totaling two million specimens. Confiscated items included live mammals, birds, and reptiles along with bodies, parts, and products. In 2012, we received and analyzed tissues from primates smuggled into JFK airport in the United States. These contained sequences of human pathogens, including four strains of simian foamy virus (an agent closely related to HIV) and two herpes viruses.

This brings us to discussion of the lab-leak speculation. Some of my colleagues believe that research with infectious agents—particularly gain-of-function research, where a virus or other microbe is genetically manipulated to alter its ability to infect cells or cause disease—should also be banned. I disagree. This type of research is needed to understand how viruses infect us and thwart our immunological defenses. It is also essential to identifying and validating effective countermeasures. However, it should only be pursued in accordance with an internationally approved standard for biocontainment, project review that considers risk/benefit analysis, rigorous training, and continuous monitoring of personnel that includes antibody testing for evidence of laboratory-acquired infections.
After 9/11 and the anthrax attacks that followed, President George W. Bush established a National Biosurveillance Advisory Subcommittee to review infectious threats. A second subcommittee was established by President Barack Obama. Both committees issued reports (see the first report and the second report) that recommended investments in new technologies and personnel for national and global surveillance of and response to emerging infectious diseases. The committees endorsed the WHO International Health Regulations of 2005 that mandated building infrastructure for detecting microbial threats in the developing world.
Neither report gathered much traction until the film “Contagion” was released in 2011. “Contagion” was developed to call attention to pandemic risks originating in wildlife. It introduced audiences worldwide to epidemiology, biosafety laboratories, the process of vaccine development, false panaceas, and the term “R naught”—which describes how many people on average someone with an infectious disease will infect. It also led The New York Times to invite me to write an editorial that laid out what was needed to prevent a pandemic. There is much that the film got right; however, we did not predict the potential for asymptomatic transmission, promiscuous infection of multiple organ systems, or the impact of poor leadership.
What would good leadership entail now? The common elements in the several roadmaps proposed since the COVID-19 pandemic began (see Foreign Affairs, The Independent Panel, and the G20 Action Plan) include a stronger World Health Organization and global investment in the research and public health resources needed for effective surveillance, along with drug and vaccine development, production, and distribution. As understood in the context of the current pandemic, it is impossible to overstate the importance of surveillance and vaccines. We would not have been caught flat-footed if we knew that a novel coronavirus was circulating in the autumn of 2019. Early access to accurate diagnostic assays and track tracing would have enabled early containment. Similarly, if effective vaccines had been distributed globally, we might never have allowed SARS-CoV-2 the opportunity to evolve into the delta variant. The virus is still adapting and new variants will continue to emerge that will challenge us. At an estimated cost of $25 billion, vaccinating the world is a bargain and an ethical imperative.
SARS-CoV-2 is now endemic worldwide in people, wildlife, and domestic animals; it is unlikely that we can eradicate it. I am nonetheless optimistic that we will ultimately bring it under control. The question is the durability of what we learn from this experience. As damaging as COVID-19 has been to humankind, there are other pathogens lurking worldwide—antibiotic-resistant bacteria, fungi, and threats to food security. Our commitment to a shared vision for global health must therefore continue even after the current pandemic recedes.
Together, we make the world safer.
The Bulletin elevates expert voices above the noise. But as an independent nonprofit organization, our operations depend on the support of readers like you. Help us continue to deliver quality journalism that holds leaders accountable. Your support of our work at any level is important. In return, we promise our coverage will be understandable, influential, vigilant, solution-oriented, and fair-minded. Together we can make a difference.
Keywords: Coronavirus, lab leak, pandemic origin, wildlife trade
Topics: Disruptive Technologies















Dogmatic belief and the cynical opportunists who seek to harness it as a tool and a weapon will doom humanity in time. The United States and much of the world is divided into those who believe what they want to believe and those that believe in evidence and science. For every person supporting or taking proactive steps toward enhanced surveillance, prevention and cures there is at least one other person licking the Petri dish or sticking their nose in the face of an infected person because the thinky-people said that they shouldn’t. It isn’t possible to convince a dogmatic believer… Read more »
I entirely agree. However, a rampaging virus, or mutation thereof, is merely one component of a broader swathe of ‘anthropogenic vectors of doom’ that face the extant denizens of this beleaguered Earth. Where the biosphere is in a state of flux, a mass extinction underway and a human populace approaching nine billion souls. Already countless self-reinforcing feedbacks have been triggered, with more to follow as the Arctic ocean becomes ice-free. As we’ve seen with the outbreak in the tundras of western Siberia, an ancient and dormant pathogen – anthrax – can be released from ancient thawing carcasses and Russian scientists… Read more »
If SARS-Cov-2 existed prior to Wuhan, China in Fall 2019, shouldn’t we expect to find ‘older’ relatives (at least as contamination) among the large number of available SARS-CoV-2 genome sequences isolated from patients from around the world?