What it means when COVID-19 antibodies fade
By Jackie Flynn Mogensen | July 24, 2020
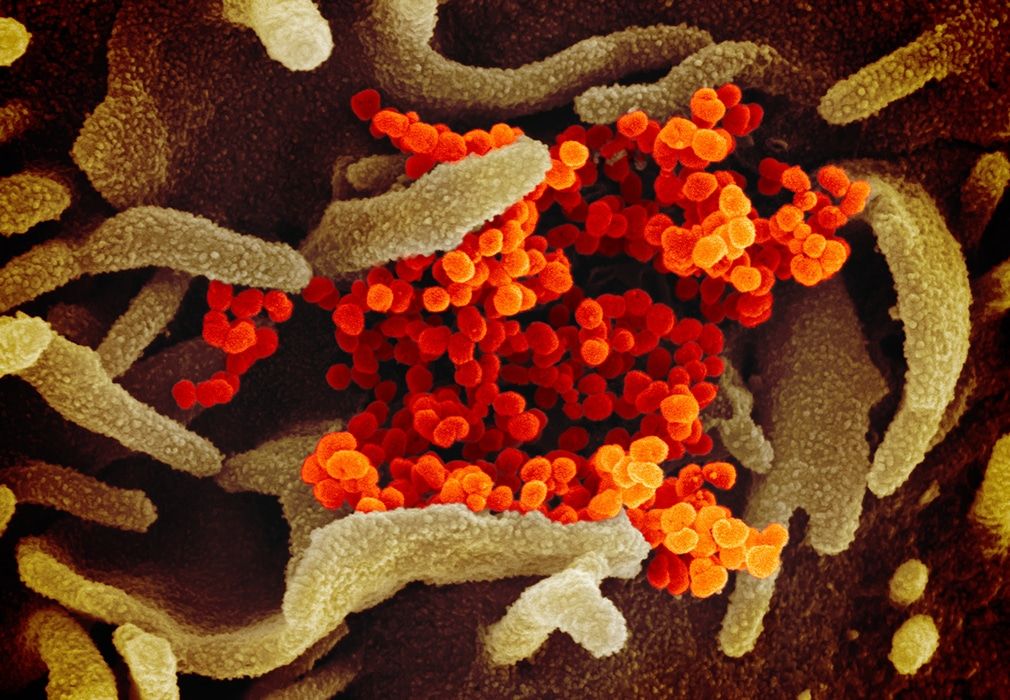 Scanning electron microscope image showing the virus that causes COVID-19 emerging from the surface of cells (green) cultured in the lab. Image captured and colorized at NIAID's Rocky Mountain Laboratories (RML) in Hamilton, Montana. Credit: NIAID
Scanning electron microscope image showing the virus that causes COVID-19 emerging from the surface of cells (green) cultured in the lab. Image captured and colorized at NIAID's Rocky Mountain Laboratories (RML) in Hamilton, Montana. Credit: NIAID
Editor’s note: This story was originally published by Mother Jones. It appears here as part of the Climate Desk collaboration.
Since the start of the pandemic, one of the biggest—and most critical—gaps in our understanding of the coronavirus has been about antibodies. Based on what we know about other coronaviruses, scientists have speculated that a person who recovers from COVID-19 would likely develop virus-fighting antibodies and may have some immunity, at least in the short term. The power of these antibodies would be crucial in protecting survivors, in reopening the economy, and perhaps even in treating patients still sick with the virus. But just how long the antibodies would last was a mystery.
Then last month, a study published in Nature Medicine delivered what seemed like bad news: Antibodies to the coronavirus may start to fade within just two-to-three months. It also suggested that people who were asymptomatic are more likely to have their antibodies drop to undetectable levels during recovery. A subsequent study led by researchers at King’s College London, published earlier this month and before peer-review, supports these findings.
While the Nature Medicine study is fairly small, focusing on a group of 37 people with symptoms, plus 37 people without, the results are an early indication that some measures, like instituting “immunity passports”—a certificate doled out to recovered (and presumably immune) COVID-19 patients—may not be feasible or safe. The results also “support the prolongation of public health interventions,” the authors write, like social distancing, hand washing, and widespread testing.
Still, as a card-carrying optimist, I’d argue that there isn’t reason to really agonize over your antibodies—at least not yet. The results, though they are not great news, are just one piece of the COVID puzzle; there’s still just so much we don’t know about immunity to this coronavirus, including whether a second COVID-19 infection is possible in either the short- or long- term. But to get a better understanding of what fading antibodies mean, for us as individuals and as a country, I called Stanley Perlman, a professor of microbiology and immunology, as well as pediatrics, at the University of Iowa Carver College of Medicine, to walk me through the science. As he explains, it’s not really a surprise that antibodies may not last for more than a few months, and it’s possible that people who have recovered from milder cases of COVID-19—even with low levels of antibodies—may still be protected from severe disease. Plus, he notes, antibodies aren’t the only method our immune system employs to fight off viruses. Here’s a brief breakdown of our conversation.
With other coronaviruses, it’s not unusual for antibodies to wane.
While we don’t truly know what “normal” is with this particular coronavirus, Perlman says, “I think our experience with many, many respiratory viral infections shows that immunity doesn’t last that long. That’s why people get colds over and over again.”
For instance, with MERS, which is caused by another coronavirus, research done by Perlman and others shows that some people who had very mild cases never developed an antibody response. Other people’s antibodies faded after a few months. And some people with severe cases saw antibodies stick around for more than a year.
In one influential study conducted in 1990, researchers purposefully exposed volunteers to cold-causing coronaviruses. Some got colds, others didn’t. A year later, when the volunteers were exposed to the same virus, most did not develop cold symptoms again, likely because their antibody levels had been boosted by the initial exposure. “But,” says Perlman, “I bet if you had waited two or three years, they would have been susceptible again.”
“There are different amounts of waning and it’s probably what respiratory viruses always do,” he says. “So what this virus is doing doesn’t appear to me any different than what I would have expected based on what I know about MERS and what I know about the common cold coronaviruses.”
Even if our antibodies wane, our immune systems may still be able to fight off the coronavirus.
“When we get infected,” explains Perlman, “the first thing a virus does is enter a cell to make more virus.”
Unlike bacteria, viruses aren’t free-floating around in our bodies for long. They shelter in our cells and replicate. But antibodies don’t typically enter cells—”that’s why they work against bacteria,” Perlman says. “If you don’t have a free virus, then the antibody will work less well.”
Luckily, we have other virus fighters in our bodies that do attack infected cells: T cells. “What T cells can do is they can recognize an infected cell and destroy it,” says Perlman. Other T cells assist in making better antibodies. “In general, you need the two [antibodies and T cells] together for effective immunity.” And when it comes to the new coronavirus, there has been some early research that indicates COVID-19 patients—and even some never infected with SARS-CoV-2—have T cells that can recognize the virus.
On top of that, even if antibody levels drop after a few months, Perlman says, the cells that make the antibody, called B cells, may still be present. “So if your [antibody] replacement occurs at a low level, and you can’t detect it anymore, but the B cells are still there, you might still be protected.”
This could mean that second infections may be less severe.
While, again, experts still aren’t sure if reinfection is possible, based on what we know about the immune system, a second infection may be less severe, Perlman says; a recovered patient, for example, may be less likely to develop pneumonia as a result of COVID-19, which is what often happens in the most severe cases of the disease. “If I’ve had the infection and my antibody wanes,” Perlman says, “I bet because of my [T cell responses], as well as these residual memory B cells, I won’t get pneumonia again.”
That said, at this early point in our understanding of the virus, it’s difficult to know much of anything definitively. And, in fact, there are some reports that appear to counter the idea that a second infection would be less severe. For instance, last week Vox published an account from a physician in Washington, DC, who describes a patient who seems to have contracted the coronavirus twice. While his first infection included a “mild cough and sore throat,” the second infection was more severe (“high fever, shortness of breath, and hypoxia, resulting in multiple trips to the hospital”). Based on this evidence, the physician argues, reinfection may be possible and that “Covid-19 may also be much worse the second time around.”
When I presented this case to Perlman via email, he wasn’t sure what to make of it. He pointed out that the patient’s immunity could have been short-lived, making him still susceptible to the virus, but that he “expected even mild disease to be somewhat protective,” adding, “this [example] would be counter to what I expected.” It’s also possible, he says, that the patient never developed an immune response to the first infection. (Notably, the patient did not receive an antibody test, so we can’t be sure.) “A mystery, but one that has been described occasionally,” Perlman noted, suggesting the report may be an outlier.
Even if you don’t get sick again, reexposure could still cause you to spread it to others.
In the volunteer study from 1990, people who were reexposed to a coronavirus that caused the common cold still “shed”—that is, released particles of the virus—even if they weren’t experiencing symptoms. The studies indicate that even someone whose immune system can fend off a full-blown infection may still release viral particles. “But what [the studies] don’t tell me is, how much will I shed the virus?” Perlman says. “And that matters because obviously, if one has had COVID-19 or has been vaccinated, that person may feel comfortable going out into public. And if he or she then gets reinfected, and they don’t get a severe case, that’s great for the individual, but we want to make sure that person isn’t shedding or producing as much virus as someone who’s never been immunized.”
The amount someone is shedding virus may impact how infectious they are. Let’s say Donald Trump has been infected with the coronavirus and Anthony Fauci hasn’t. If they both pick up the virus—for Trump, a second time, and for Fauci, the first, in this hypothetical scenario—Fauci may end up shedding a lot more virus than Trump because the president’s immune system would have seen the virus before, and was, therefore, more prepared to fight it. A person shedding more virus is likely “much, much, much more infectious” than the other, Perlman says.
This uncertainty means we should be careful about “immunity passports.” “When people talk about immunity certificates, one has to be so careful,” Perlman says. “You may have sufficient immunity to protect you from pneumonia, but you may not have enough immunity to protect me from getting infected [with COVID-19] by you if you’re still shedding the virus.” Therefore, he says, introducing immunity passports may be “premature.”
We don’t know yet how fading antibodies could affect herd immunity.
“This is part of the whole confusing story,” Perlman says. “If the antibody goes away, that’s not good. But on the other hand, if when you see a pathogen again, if your antibody quickly comes back, and now you’re protected, that’s good. That’s where we don’t really have that much information.”
Still, Perlman notes, controlling the epidemic is ultimately about saving lives. So even if people can become reinfected, if they aren’t getting severely ill and aren’t highly contagious, it’s possible that a form of herd immunity is achievable. It all depends on how contagious people are if they can become reinfected. “If most people are protected from severe pneumonia, then we can say the epidemic is under control. Because if you’re protected, and now suddenly you’re exposed to me, and you get cold-like symptoms, you may not like getting your cold but as long as everyone around you has the same situation, then we all get colds. And that is what it is, but it’s not terrible.”
“Even if our antibodies drop to undetectable levels,” Perlman adds, “I would bet that if we got that 70 percent of the population, we’d be protected. I don’t know that for sure. But I bet you that there’ll be some sort of protection that’ll be equivalent to herd immunity.”
We also don’t know what fading antibodies will mean for vaccine development.
Some researchers have speculated that if antibodies diminish, protection from a vaccine may do the same. “People do have antibodies, but the antibodies are waning quickly,” one researcher recently told the San Francisco Chronicle. And if antibodies diminish, “then there is a good chance the immunity from a vaccine would wane too.”
While it’s true that waning immunity—naturally or from a vaccine—isn’t ideal, Perlman says, remember that we don’t yet have a clear picture of how much antibodies fade. Just because immunity drops doesn’t mean it will go away entirely. Plus, our immune systems may respond differently to a vaccine versus a natural infection: “We’re pretty sure, at least based on other coronavirus infections, if a person has a severe infection, then their immunity won’t wane so much. If they have a mild infection, it might wane. We don’t know what a vaccine will do: Will it be more like a mild infection or a severe infection? And that’s really the critical question.”
There are at least four key questions we still need answers to.
When I asked Perlman what we still need to determine about antibodies and reinfection, he laid out the following lines of inquiry:
- “How much waning [of antibodies] really occurs?”
- “How much does this relate to the severity of the initial infection?” In other words, do people with the most severe cases mount a better immune response, as we saw with MERS?
- “In people who have had waning immunity, can they get severe pneumonia?” In other words, do they ever get really sick again?
- “Do they shed anything—RNA or virus?” Someone shedding virus would be infectious, while a person shedding just RNA—remnants of the virus—wouldn’t be infectious. “That’s what I’d want to know.”
Together, we make the world safer.
The Bulletin elevates expert voices above the noise. But as an independent nonprofit organization, our operations depend on the support of readers like you. Help us continue to deliver quality journalism that holds leaders accountable. Your support of our work at any level is important. In return, we promise our coverage will be understandable, influential, vigilant, solution-oriented, and fair-minded. Together we can make a difference.
Keywords: COVID-19, Coronavirus, antibodies, disease resistance, pandemic
Topics: Biosecurity, Special Topics














I think whether a recovered patient with a waning antibody will get mild or severe symptoms from re-exposure of the coronavirus depends largely on how much memory T cells and memory B cells are circulating inside their body
First question: By what means is “infection” determined. PCR tests do not confirm infection, and unless viral culture was done, we have no idea whether , in fact. any infection occurred.
Only in established cases (culture proven) can we begin to investigate antibody presence.
Our Immune system has built in within it the development of long term memory, we get repeat bouts of the flu because the viruses mutate, not because of the immune sytems failings. We use vaccinations to develop immunity, otherwise it would serve no purpose.