Charging ahead: Steven Chu, Nobel Prize-winner and former Energy Secretary, on today’s battery research—and more
By Dan Drollette Jr | November 8, 2023
Charging ahead: Steven Chu, Nobel Prize-winner and former Energy Secretary, on today’s battery research—and more
By Dan Drollette Jr | November 8, 2023
Steven Chu won a Nobel Prize in physics in 1997 for his earlier work on cooling and trapping atoms with laser light at the legendary Bell Laboratories. He then went on to become the US Secretary of Energy—after serving as director of the Lawrence Berkeley National Laboratory. After finishing his term as secretary, Chu went back to the lab bench at Stanford University, where he has been exploring batteries and renewable energy technology, among other lines of research.
Chu has long been a vocal advocate for more research into renewable energy and nuclear power, arguing that a shift away from fossil fuels is essential if we are to combat climate change.
In this interview with the Bulletin’s Dan Drollette Jr, Chu talks about the holy grail of battery research—the solid-state battery—and why researchers are so eager to develop it. He also discusses the roles of rare earth metals, carbon sequestration, the Inflation Reduction Act, and the quirks of the economics of the auto industry. In the process, he explains why the full charge of a car battery only needs to be long enough to go about 200 miles at a time: “You don’t really need anything more than that; the goal is simply to make the battery last longer than the human bladder.”
(Editor’s note: This interview has been condensed and edited for brevity and clarity.)

Dan Drollette Jr: The last time I interviewed you,[1] you’d said that after winning the Nobel Prize and after being the Secretary of Energy, you wanted to get back in the lab and do research on batteries and renewable energy. So I thought you’d be the perfect person to ask about this big breakthrough that Toyota just announced.[2] They said they had developed a new kind of electric car battery that can go from dead flat to fully charged in 10 minutes—and that the battery could go 745 miles on a single charge.
Steven Chu: Okay, so, first, I am indeed working on batteries. [3][For an explanation of battery basics, see endnote 3.]
And a little bit on lithium extraction. Also, we’re trying to look for radically new ways of capturing carbon—not only from point sources, but also from the atmosphere. And I also spend about a half or a third of my time trying to influence policymakers, and work on advisory boards. In my department at Stanford, I have about eight people, with one or two people working in each area.
But to get back to the news—what Toyota said is surprising. I know that researchers have been talking about aiming for an average range of about 400 miles, with the idea that to do that you’d probably be charging the battery from a low of 25 percent to a high of 75 percent. That’s the sweet spot; in those mid-ranges, you can charge three or four times faster than if you were to go from a low of 20 percent up to 95-percent charged. And those percentages are important, because those last few percent at either end are the hardest—you never want to run a battery down completely, as that cuts down a battery’s life tremendously. At the same time, you don’t really get all that much more by charging a battery to the max, because you run into the law of diminishing returns.
Now, if you have a big enough battery, with enough capacity to withstand the heat that is generated, then you can swing from extreme to extreme like that—sort of. But it’s really not needed. And I’m speaking as someone who is on the board of a battery company. We know what the auto manufacturers, also known as OEMs [original equipment manufacturers], are looking for: They want to get a battery mostly charged, in a reasonable amount of time; a good rule of thumb is 80-percent charged in less than 20 minutes.
So that part of their announcement holds up. The only thing I don’t understand is this 745-mile range.[4]
Because as I said before, that requires a very big battery—which means very heavy. And you wouldn’t want to do that, because that’s a lot of extra weight to haul around. I read a thing from the International Energy Agency[5] that said that when batteries get so big and heavy, that starts to defeat the purpose. And that sheer weight does more than just hurt the purchaser of the vehicle; at some point, it becomes detrimental to the roads. To give you an idea of how much weight we’re talking about, the battery system for a Tesla Model S weighs somewhere between 1,200 and 1,400 pounds for the whole battery package—including the cooling systems and everything else—and the battery often makes up as much as a quarter of an EV’s overall weight.[6]
Now, 1,400 pounds is simply a lot of weight for what is called the traction battery—the battery that actually makes the car move, as opposed to the small 12-volt battery that runs things like the headlights and the radio.
And who needs to go 745 miles on a single charge to a traction battery? Most people do not travel more than a couple hundred miles a day, unless you’re a traveling salesman or someone who lives in an extremely remote area where there are long distances between destinations.
But even if you’re driving from LA to San Francisco—which is a four- or five- hour drive—you’d probably want to rest up anyway. And you can use that break at the rest stop to charge up the traction battery.
I’ve been kind of watching this field and, you know, it’s moving along, and samples are beginning to be sent out. All sorts of rumors about Toyota have been going on for at least half-a-dozen years now, about how they’re on the verge of a solid-state battery—but the unveiling keeps getting continually pushed back. So I don’t know how close they are to a solid-state electrolyte battery.[7]
Drollette: What is a reasonable target for the number of miles someone can go on a single charge?
Chu: I’d say 350 miles is adequate, especially if you have the capacity to do a fast charge—meaning that after only charging five or six minutes, you can go 150 miles or 200 miles. You don’t really need anything more than that; the goal is simply to make the battery last longer than the human bladder. As I said, you’re going to need a break anyway after a few hours, so you can put that rest stop to good use and charge your car’s battery at the same time.
Drollette: And how long should it take to charge the battery up to the 80-percent mark?
Chu: If it takes four or five minutes, that’s ideal, because you as the consumer don’t really care how long it takes to charge with that kind of time frame.
And as a matter of fact, it turns out that the service stations at those highway rest stops would like you to spend at least four or five minutes at their facility—because while you’re there, you tend to go in their store and buy things like soda and magazines and beer, and they make much more profit selling stuff like that than they do selling gas.
Selling the gasoline itself has razor-thin profit margins; they make a lot more on these retail sales. And so if they can have you loiter around for a few minutes, then they stand that much more chance of you buying something.
But at the same time, they don’t want you to linger too long; if you take something like 20 minutes or more, then your car gets in the way of other cars. So, it’s more than coincidence that when it comes to how long it takes to charge your electric car battery, the industry is aiming for 10—or at most 20—minutes to get it mostly charged.
To some extent, without overstating the case, the gasoline is like a loss leader; the idea is to just get people into the gas station’s convenience store and get them to buy overpriced candy bars. It’s a lot like the retail sales of cars: OEMs make most of their money on servicing the car and not so much on the auto sales themselves.
To give you an example, my wife recently took our old car in to the dealership, and by the time they were done with servicing that car—changing the timing belt and things like that—the cost was about equal to the car’s Blue Book value. Admittedly, the car is now 22 years old, so it didn’t take much to get to that value. But that gives you an idea of the economics of this business.
Drollette: I read something, I think in the Green Car Journal, that said that the average internal combustion engine has something like 2,000 moving mechanical parts, while the average electric car has fewer than 200.
Chu: Right. Different sources vary on the exact number, but there seems to be general agreement that the average EV has about one-tenth the number of moving parts of a gasoline-powered vehicle, so you need a lot less maintenance for the typical electric car.
And the OEMs know that this source of income—maintenance—may dry up. Which is one reason why OEMs—automakers—are looking at other ways to structure their business. They are beginning to look at the way Tesla has marketed their cars, which is directly to the consumer.
But to get back to the battery: The batteries are getting better. And the amount of energy they can store per unit volume—their energy density—is going up dramatically. There is at least a 50-percent higher energy density in the works, and they may even be able to double that in 10 or 15 years.
Drollette: I wanted to follow up on something you touched on briefly earlier. You said that Toyota always seems to be on the verge of a breakthrough; there’s always something in the rumor mill. That’s not to pick on Toyota; but to outsiders like me, it seems like there’s always something big about to be happening, regardless of manufacturer.
Chu: That’s true, especially with batteries. They’re always claiming that they’re on the verge of a solid-state electrolyte battery, which would be safer and less flammable. And those problems have always been a concern, because the mobility of the lithium ions is lower.
There’s a couple of startup companies, with very high valuations, that have been trying to get around this. But they haven’t made deliveries on solid-state batteries, so far.
But there are actually some engineering fixes coming down the pike. The first approach would be to keep the whole battery warm. But then people found that you don’t really have to do that—you just need to keep the lithium warm, right at the electrolyte level. And there is a company coming out with a patent on that. And that means that you would just need a tickle of energy to keep the interface warm, which is where the lithium mobility often dies.[8]
So these things are coming along.
But getting around the liquid electrolytes that are commonly used is one of the real targets, because those electrolytes are so flammable. When you get a fire in a lithium-ion battery, it’s usually the electrolyte base—the thing that carries the lithium—that catches fire first.
Drollette: So in general, your feeling is that we’re getting closer to solid-state?
Chu: Yes, kind of. But let’s back out and talk about evolutionary stuff.
First, they started with lithium in graphite—the first Sony battery—which earned its creators the Nobel Prize. Using graphite as a medium made them much safer.
But with lithium and graphite, that’s roughly a maximum of seven carbon atoms for one lithium ion. And so you’re paying a lot of overhead.
Now, the next revolution—or evolution—would be bits of silicon in place of graphite. If you have silicon you can get roughly four lithiums around each silicon atom. So even though silicon weighs more, you’ve upended the ratio tremendously; you are increasing the energy density by roughly half. And that’s why you can talk about going from 250 watt-hours per kilogram to maybe 400. Because these are going to be high content silicon.
Beyond that, the next holy grail would be pure metal—then there’s no overhead.
Drollette: Is the supply of precious metals—such as lithium, cobalt, rhodium—a significant choke point?
Chu: It will be, for sure. And even nickel will be, for that matter. Because I’m anticipating that in 10 or 20 years, 80 percent of new car sales will be electric vehicles. A lot of cities, if not countries, are going to be ruling out internal combustion engine cars; there will only be EVs and hybrids. And so all of a sudden, the market share for batteries will go way up.
Meanwhile, the price of batteries is really dropping phenomenally; it’s a lot like what happened with silicon solar cells in the 1970s or ‘80s. It’s amazing.
Drollette: I guess there’s a tendency to interpret this information about the scarcity of rare earth minerals to mean that it’s going to stop the transition to electric vehicles in its tracks.
Chu: Well, they’re rare—but not that rare. [9] It’s really the separating them out that is the problem; it’s such a polluting process.
Although I will note that China, for one, has been very strategically locking up contracts for minerals that will be at the heart of the technologies of the future: they’re buying up lithium rights in Australia, cobalt in Nigeria, and so forth, and then bringing them all to China for processing. That way, they’ve got the key materials for manufacturing their own cars and whatnot in the future.
But rare earth metals are not a showstopper by any means. Rares are not that rare—and in any case, the market will respond to this, and people will develop substitutes. It’s mostly a pollution and refining issue. And even China has this problem.
Drollette: Speaking of which, I’m curious to hear what you think about Biden’s $370 billion clean energy efforts.
Chu: They call it the “Inflation Reduction Act,” but yeah, it was a clean energy bill.
Drollette: Is the US getting close to the equivalent of Germany’s energiewende, the “soft energy path?” Is this bill effective in encouraging the kinds of investments needed to switch from fossil fuels to renewables?
Chu: Yes and no. I think half of the Inflation Reduction Act consists of tax breaks, which are easier to administer. They’re not grants or subsidies, things like that. But my overall feeling is that it is making progress.
It’s not like an overnight change; it’s not that dramatic. And if I were in charge, I probably would not have subsidized oil and gas as much as they did. But these things are never easy, as we saw with what Germany did. I advise the Helmholtz folks[10] on their energy programs, and a lot of their strategy was just to use cheap Russian gas—and we all know how that worked out.
Now, they seem to have pivoted from that, but they’re ultimately still trying to figure out how to be environmentally friendly while keeping German energy prices low enough that their heavy industry doesn’t fall short. If Germany’s energy prices go too high, then their plastics, steel, automobile manufacturing, and other economic bases will go elsewhere, where energy is much cheaper. And the same could be said for Japan, South Korea, and other advanced nations.
Drollette: Before we wrap up—you said you’re doing research into carbon capture and sequestering. Are there any comments you’d like to make?
Chu: Sure. So let me start with the fact that I think we’re going to need carbon capture for a whole bunch of things. The price of doing it is high, about a couple hundred dollars a ton or more for air capture, depending on who you talk to. But people are not going to do capture carbon commercially unless the price is like $100. Right? So, you don’t break even. But despite the dismal numbers, carbon capture is going to be needed, no matter what.
Now, there are some people who say “no” to the very idea of carbon capture and sequestration, arguing that if you have carbon capture, then you encourage the fossil fuel industry to continue doing business as usual. But I feel that releasing carbon is such a basic byproduct of every activity we do, that it’s inevitable. There are so many other sources of carbon out there—manufacturing steel, cement, plastics—in addition to the emissions from power plants that are fossil-fueled.
The long-term prospects of more greenhouse gas are going to be very, very bad, according to the climate models—and a lot of it is already baked-in and still to come. Because the oceans are very cold and surface water/deep water mixing is very slow we won’t see the full effects of all the carbon we’ve already pumped into the atmosphere for decades, maybe a half-century.
So we’ve got to get that carbon out; we can’t just stop the average global temperature from continuing to rise. Because we’ve got to actually stop the things that we have already set in motion.
Consequently, it’s absolutely necessary to remove the existing carbon we’ve already pumped into the atmosphere. Even if we get rid of fossil fuel for electricity generation, there’s 25 percent of greenhouse gas emissions that comes from agriculture—food production, food waste, and the raising of animals. Not to mention all the carbon that comes from transportation, everything.
When I was head of Lawrence Berkeley National Laboratory, and then Secretary of Energy, I became convinced that carbon capture was a necessary technology. And not just to keep the fossil fuel industry from going away. We’ll still need carbon capture.
Drollette: When you ended your time at the Department of Energy, you gave a speech that warned about the risks of a continued reliance on fossil fuels.
Chu: Yes, I said something to the effect that the Stone Age did not end because we ran out of stones, it ended because we transitioned to better solutions. And similarly, the oil age will come to an end, but not for lack of oil. It will come to an end because fossil fuels have profound effects on the climate. Carbon has tremendous social costs, in the form of weather extremes, extinctions, and air pollution.
Electric vehicles have so many advantages; they allow us to transition to a better solution. I used to joke that if you look around, you see a lot of stones in the ground—but you don’t say to yourself: “If it weren’t for a bunch of tree huggers, we wouldn’t have these stranded assets.”
Endnotes
[1] See “Taking stock: Steven Chu, former secretary of Energy, on fracking, renewables, nuclear weapons, and his work, post-Nobel Prize,” Dan Drollette Jr, Bulletin of the Atomic Scientists, November 1, 2016.
[2] See “Toyota claims battery breakthrough in potential boost for electric cars,” by Rob Davies, The Guardian, July 4, 2023,
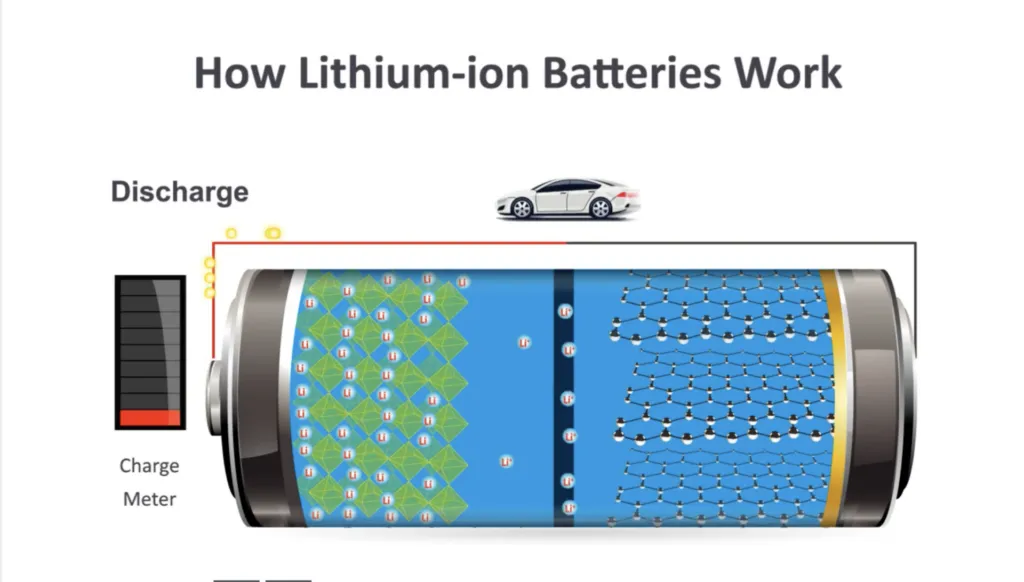
[3] A battery is a device that is able to store electrical energy in the form of chemical energy, and then convert that energy back into electricity when called upon. The chemical reactions in a battery involve the flow of electrons from one material (known as an anode) to another (known as a cathode), through an external circuit. This flow of electrons provides an electric current that can be used to do work—whether it be moving a car, operating a cell phone, or powering a laptop. To enable the electrons to move within the battery, they are carried by a liquid known as an electrolyte solution that is in contact with both the anode and cathode. Anodes and cathodes made from different substances produce different chemical reactions that affect how the battery works. In other words, what the anodes and cathodes are made of affects how much energy the battery can store and its voltage. For more, see “How a battery works.”
[4] See “The obsession with EV range is all wrong,” by Shannon Osaka, The Washington Post, July 7, 2023.
[5] See “Global EV Outlook 2023: Trends in Batteries,” International Energy Agency.
[6] For more, see “Electric car battery weight explained,” EV Box Blog.
[7] As the name implies, a solid-state battery would be just that—a battery that does not use a liquid electrolyte solution to ferry the ions that make for a charge, such as what a lithium-ion battery does. A solid-state battery can also store more energy, pound for pound, than a battery that is liquid-based, and it does not run the same risks of overheating. It would also have more range and charge twice as fast. But this new technology is still very much in the R&D phase.
[8] According to energy.gov, the battery cell of a lithium-ion battery—the most common one used in cars and many other devices—is made up of an anode, a cathode, a separator, electrolytes, and two current collectors (positive and negative). The anode and cathode store the lithium. The electrolyte carries positively charged lithium ions from the anode to the cathode—and vice versa —through the separator. The movement of the lithium ions creates free electrons in the anode which creates a charge at the positive current collector. The electrical current then flows from the current collector through the device being powered (car, cell phone, computer, etc.) to the negative current collector. The separator blocks the flow of electrons inside the battery.
Multiple individual lithium-ion battery cells are then connected to make a battery module. A group of connected battery modules is then contained within an enclosed battery casing with underbody protection. This is known as the battery pack—which is the big heavy traction battery that you can see if you crawl under an electric vehicle or a hybrid. On early Toyota Prius hybrids, for example, the traction battery pack is in the form of a rectangular shape roughly about 16 inches wide by 34 inches long and 8 inches deep. Its capacity is rated in kilowatt-hours.
[9] For more on these elements, see “Electric Vehicles, Batteries, Cobalt, and Rare Earth Metals” by Josh Goldman, Union of Concerned Scientists, October 25, 2017.
[10] The Helmholtz Association of German Research Centers is the largest scientific organization in Germany. A union of 18 scientific-technical and biological-medical research centers, the association’s official mission is “solving the grand challenges of science, society and industry.”
Together, we make the world safer.
The Bulletin elevates expert voices above the noise. But as an independent nonprofit organization, our operations depend on the support of readers like you. Help us continue to deliver quality journalism that holds leaders accountable. Your support of our work at any level is important. In return, we promise our coverage will be understandable, influential, vigilant, solution-oriented, and fair-minded. Together we can make a difference.
Keywords: EV, Energy Department, Steven Chu, batteries, climate change, electric vehicles, renewable energy
Topics: Climate Change